Publications
2023
Titanium Salalen Catalyzed Enantioselective Benzylic Hydroxylation
C. Wartmann, S. Nandi, J.-M. Neudörfl, A. Berkessel Angew. Chem. Int. Ed. 2023, e202306584.
DOI (English Version): 10.1002/anie.202306584
Hydrogen Bonding Shuts Down Tunneling in Hydroxycarbenes: A Gas-Phase Study by Tandem-Mass Spectrometry, Infrared Ion Spectroscopy, and Theory
M. Paul, T. Thomulka, W. Harnying, J.-M. Neudörfl, C. R. Adams, J. Martens, G. Berden, J. Oomens, A. J. H. M. Meijer, A. Berkessel, M. Schäfer, J. Am. Chem. Soc. 2023, 145, 12124-12135.
DOI: 10.1021/jacs.3c01698
Novel Gold(I) Complexes Induce Apoptosis in Leukemia Cells via the ROS-Induced Mitochondrial Pathway with an Upregulation of Harakiri and Overcome Multi Drug Resistances in Leukemia and Lymphoma Cells and Sensitize Drug Resistant Tumor Cells to Apoptosis in vitro
M.-C. Ahrweiler-Sawaryn, A. Biswas, C. Frias, J. Frias, N. L. Wilke, N. Wilke, A. Berkessel, A. Prokop Biomedicine & Pharmacotherapy 2023, 262, 114507.
DOI: 10.1016/j.biopha.2023.114507
2022
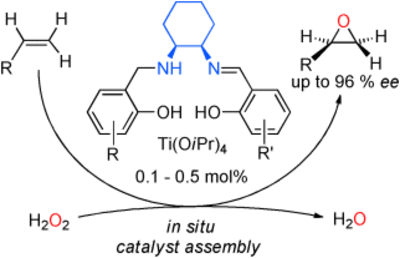
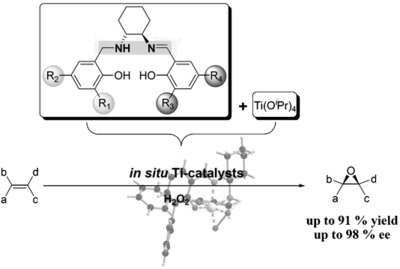
syn-Selective Epoxidation of Chiral Terminal Allylic Alcohols with a Titanium Salalen Catalyst and Hydrogen Peroxide
F. Severin, G. M. Fusi, C. Wartmann, J.-M. Neudörfl, A. Berkessel Angew. Chem. Int. Ed. 2022, 61, e202201790.
DOI (English Version): 10.1002/anie.202201790
DOI (Deutsche Version): 10.1002/ange.202201790
Very Important Paper
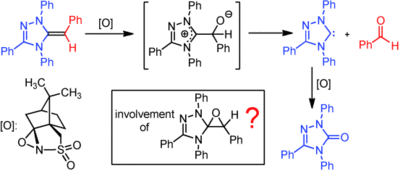
Formation of Breslow Intermediates from N-Heterocyclic Carbenes and Aldehydes Involves Autocatalysis by the Breslow Intermediate, and a Hemiacetal
A. Wessels, M. Klußmann, M. Breugst, N. E. Schlörer, A. Berkessel Angew. Chem. Int. Ed. 2022, 61, e202117682.
DOI (English Version): 10.1002/anie.202117682
DOI (Deutsche Version): 10.1002/ange.202117682
Hot Paper
2021
Metabolite Identification Using Infrared Ion Spectroscopy─Novel Biomarkers for Pyridoxine-Dependent Epilepsy
R.E. van Outersterp, U.F.H. Engelke, J. Merx, G. Berden, M. Paul, T. Thomulka, A. Berkessel, M.C.D.G. Huigen, L.A.J. Kluijtmans, J.Mecinović, F.P.J.T. Rutjes, C.D.M. van Karnebeek, R.A. Wevers, T.J. Boltje, K.L.M. Coene, J. Martens, and J. Oomens Anal. Chem.2021, 93, 15340–15348.
DOI: 10.1021/acs.analchem.1c02896
N-Heterocyclic Carbene/Carboxylic Acid Co-Catalysis Enables Oxidative Esterification of Demanding Aldehydes/Enals, at Low Catalyst Loading
W. Harnying, P. Sudkaow, A. Biswas, A. Berkessel Angew. Chem. Int. Ed. 2021, 60, 19631-19636; Angew. Chem. 2021, 133, 19783-19788.
DOI (English Version): 10.1002/anie.202104712
DOI (Deutsche Version): 10.1002/ange.202104712
Hot Paper
A Metal-Free salalen Ligand with Anti-Tumor and Synergistic Activity in Resistant Leukemia and Solid Tumor Cells via Mitochondrial Pathway
S. M. Hopff, Q. Wang, C. Frias, M. Ahrweiler, N. Wilke, N. Wilke, A. Berkessel, A. Prokop J. Cancer Res. Clin. Oncol. 2021, 147, 2591–2607.
DOI: 10.1007/s00432-021-03679-3
Titelbild zu
Olefin Epoxidation Catalyzed by Titanium–Salalen Complexes: Synergistic H2O2 Activation by Dinuclear Ti Sites, Ligand H-Bonding, and π-Acidity
Hauke Engler, Markus Lansing, Christopher P. Gordon, Jörg-M. Neudörfl, Mathias Schäfer, Nils E. Schlörer, Christophe Copéret, and Albrecht Berkessel* ACS Catal. 2021, 11, 3206-3217.
Link zum Cover: https://pubs.acs.org/toc/accacs/11/6
Olefin Epoxidation Catalyzed by Titanium–Salalen Complexes: Synergistic H2O2 Activation by Dinuclear Ti Sites, Ligand H-Bonding, and π-Acidity
Hauke Engler, Markus Lansing, Christopher P. Gordon, Jörg-M. Neudörfl, Mathias Schäfer, Nils E. Schlörer, Christophe Copéret, and Albrecht Berkessel* ACS Catal. 2021, 11, 3206-3217.
DOI: 10.1021/acscatal.0c05320
Sensitizing Multidrug-Resistant Leukemia Cells to Common Cytostatics by an Aluminium Complex that has High-Apoptotic Effects in Leukemia, Burkitt Lymphoma and Mamma Carcinoma Cells
S. M. Hopff, L. A. Onambele Abodo, M. Brandenburg, A. Berkessel, A. Prokop BioMetals 2021, 34, 211–220.
DOI: 10.1007/s10534-020-00273-x
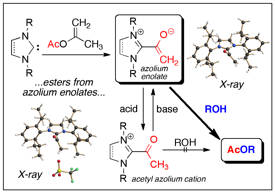
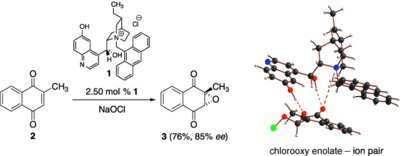
Acyl Donor Intermediates in N-Heterocyclic Carbene Catalyzed Transformations: Acyl Azolium or Azolium Enolate?
A. Biswas, J.-M. Neudörfl, N. E. Schlörer, A. Berkessel Angew. Chem. Int. Ed. 2021, 60, 4507-4511; Angew. Chem. 2021, 133, 4557-4561.
DOI (English Version): 10.1002/anie.202010348
DOI (Deutsche Version): 10.1002/ange.202010348
Enantioselective Bifunctional Ammonium Salt‐Catalyzed Syntheses of 3‐CF3S‐, 3‐RS‐, and 3‐F‐Substituted Isoindolinones
A. Eitzinger, J. Otevrel, V. Haider, A. Macchia, A. Massa, K. Faust, B. Spingler, A. Berkessel, M. WaserAdv. Synth. Catal. 2021, 363, 1955-1962.
DOI: 10.1002/adsc.202100029
Salicylic Diamines Selectively Eliminate Residual Undifferentiated Cells from Pluripotent Stem Cell-Derived Cardiomyocyte Preparations
K. Burkert, H. Taheri, S. Hamad, M. Oliverio, G. Peinkofer, J.-W. Kornfeld, W. Harnying, K. Pfannkuche, J. Hescheler, A. Berkessel*, T. Šarić Scientific Reports 2021, 11, 2391.
DOI: 10.1038/s41598-021-81351-z
Titelbild zu
Breslow Intermediates (Aminoenols) and their Keto Tautomers: First Gas‐Phase Characterization by IR Ion Spectroscopy
M. Paul, K. Peckelsen, T. Thomulka, J. Martens, G. Berden, J. Oomens, J.-M. Neudörfl, M. Breugst, A. J. H. M. Meijer, M. Schäfer, A. Berkessel Chem. Eur. J. 2021, 27, 2552.
DOI: 10.1002/chem.202004678
Very Important Paper
Breslow Intermediates (Aminoenols) and their Keto Tautomers: First Gas‐Phase Characterization by IR Ion Spectroscopy
M. Paul, K. Peckelsen, T. Thomulka, J. Martens, G. Berden, J. Oomens, J.-M. Neudörfl, M. Breugst, A. J. H. M. Meijer, M. Schäfer, A. Berkessel Chem. Eur. J. 2021, 27, 2662-2669.
DOI: 10.1002/chem.202003454
Very Important Paper
2020
Efficient epoxidation over dinuclear sites in titanium silicalite-1
C. P. Gordon, H. Engler, A. S. Tragl, M. Plodinec, T. Lunkenbein, A. Berkessel, J. H. Teles, A.-N. Parvulescu & C. Copéret Nature 2020, 586, 708-713.
DOI: 10.1038/s41586-020-2826-3
Nature News & Views 10.1038/d41586-020-02942-w
40 Jahre alter Katalysator birgt Überraschungen für die Wissenschaft/
A 40-year-old catalyst unveils its secrets
Link Pressemitteilung der UzK
Link Press releases of UzK
Discovery of a cobalt (III) salen complex that induces apoptosis in Burkitt like lymphoma and leukemia cells, overcoming multidrug resistance in vitro
S. M. Hopff, L. A. Onambele, M. Brandenburg, A. Berkessel, A. Prokop Bioorg. Chem. 2020, 104, 104193.
DOI: 10.1016/j.bioorg.2020.104193
Chemical Modification of SBS Star Block Copolymer for Templating Nanostructures in Epoxy Resin Blends
R. Pandit, R. Lach, W. Grellmann, G. H. Michler, S. Henning, J. M. Saiter, A. Berkessel, R. Adhikari Materials Today: Proceedings 2020, 29, 1156-1160.
DOI: 10.1016/j.matpr.2020.05.398
Organoarsenic Compounds with In Vitro Activity against the Malaria Parasite Plasmodium falciparum
S. Basova, N. Wilke, J. C. Koch, A. Prokop, A. Berkessel, G. Pradel, C. J. Ngwa Biomedicines 2020, 8, 260.
DOI: 10.3390/biomedicines8080260
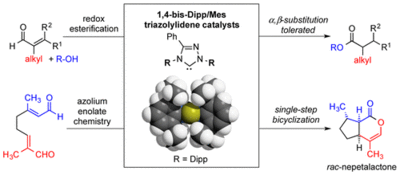
Enantiospecific Synthesis of Nepetalactones by One-Step Oxidative NHC Catalysis
W. Harnying, J.-M. Neudörfl, A. Berkessel Org. Lett. 2020, 22, 2, 386-390.
DOI: 10.1021/acs.orglett.9b04034
2019
Technical Synthesis of 1,5,9-Cyclododecatriene Revisited: Surprising Byproducts from a Venerable Industrial Process
F. Thrun, V. Hickmann, C. Stock, A. Schaefer, W. Maier, M. Breugst, N. E. Schloerer, A. Berkessel, J. H. Teles J. Org. Chem. 2019, 84, 21, 13211-13220.
DOI: 10.1021/acs.joc.9b01633
ACS Editors' Choice
Hydrogen Tunneling Avoided: Enol-Formation From a Charge-tagged Phenyl Pyruvic Acid Derivative Evidenced by Tandem-MS, IR Ion Spectroscopy and Theory
M. Paul, K. Peckelsen, T. Thomulka, J. Neudörfl, J. Martens, G. Berden, J. Oomens, A. Berkessel, A. J. H. M. Meijer and M. Schäfer Phys. Chem. Chem. Phys. 2019, 21, 16591-16600.
DOI: 10.1039/C9CP02316J
Breslow Intermediates from a Thiazolin‐2‐ylidene and Fluorinated Aldehydes: First XRD Characterization and Solution Phase NMR
M. Paul, J.-M. Neudörfl, A. Berkessel, Angew. Chem. 2019, 131, 10706-10710; Angew. Chem. Int. Ed. 2019, 58, 10596-10600.
DOI (English Version): 10.1002/anie.201904308
DOI (Deutsche Version): 10.1002/ange.201904308
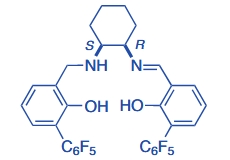
Titanium Salalen Catalysts for the Asymmetric Epoxidation of Terminal (and Other Unactivated) Olefins with Hydrogen Peroxide
A. Berkessel Aldrichim. Acta 2019, 52, 23-31.
Link: PDF-Version
Asymmetric Organocatalytic Aldol Reaction of a Hydrophobic Aldehyde in Aqueous Medium Running in a Flow Mode
L. Schober, S. Ratnam, Y. Yamashita, N. Adebar, M. Pieper, A. Berkessel, V. Hessel, H. Gröger Synthesis 2019, 51, 1178-1184.
DOI: 10.1055/s-0037-1610404
Intermediates of N-Heterocyclic Carbene (NHC) Dimerization Probed in the Gas Phase by Ion Mobility Mass Spectrometry: C-H···꞉C Hydrogen Bonding vs. Covalent Dimer Formation
M. Paul, E. Detmar, M. Schlangen, M. Breugst, J.-M. Neudörfl, H. Schwarz, A. Berkessel, M. Schäfer Chem. Eur. J. 2019, 25, 2511-2518.
DOI: 10.1002/chem.201803641
2018
Breslow Intermediates from Aromatic N-Heterocyclic Carbenes (Benzimidazolin-2-ylidenes, Thiazolin-2-ylidenes)
M. Paul, P. Sudkaow, A. Wessels, N. E. Schlörer, J.-M. Neudörfl, A. Berkessel Angew. Chem. 2018, 130, 8443–8448; Angew. Chem. Int. Ed. 2018, 57, 8310–8315.
DOI (English Version): 10.1002/anie.201801676
DOI (Deutsche Version): 10.1002/ange.201801676
Metal-Free Salan-Type Compound Induces Apoptosis and Overcomes Multidrug-Resistance in Leukemic and Lymphoma Cells in vitro
M. Dragoun*, T. Günther, C. Frias, A. Berkessel*, A. Prokop* J. Cancer Res. Clin. Oncol. 2018, 144, 685–695.
DOI: 10.1007/s00432-018-2592-x

The Rickiols, 20-, 22-, and 24-Membered Macrolides from the Ascomycete Hypoxylon rickii
F. Surup, E. Kuhnert, A. Böhm, T. Pendzialek, D. Solga, V. Wiebach, H. Engler, A. Berkessel, M. Stadler, M. Kalesse Chem. Eur. J., 2018, 24, 2200–2213.
DOI: 10.1002/chem.201704928
2017
Epoxidation of Alkenes
A. Berkessel, H. Engler, T. M. Leuther Science of Synthesis: Catalytic Oxidation in Organic Synthesis, (2017) 1, 245–307.
DOI: 10.1055/sos-SD-225-00134
Differentiation of Folate-Receptor-Positive and Negative Cells Using a Substrate Mimicking Fluorescent Probe
K. Pal, A. Heinsch, A. Berkessel, A. L. Koner Chem. Eur. J., 2017, 23, 15008–15011.
DOI: 10.1002/chem.201703305
Asymmetric Cyclopropanation of Olefins Catalyzed by a Chiral Cobalt(II) Porphyrin
A. Berkessel, E. Ertürk, J.-M. Neudörfl Org. Commun., 2017, 10, 79–89.
DOI: 10.25135/acg.oc.11.17.04.019
Kinetic Resolution of 5-Substituted Oxazinones with Bifunctional Chiral Base/Squaramide Organocatalysts
S. Eröksüz, J.-M. Neudörfl, A. Berkessel Synlett, 2017, 28, 1278–1281.
DOI: 10.1055/s-0036-1588852
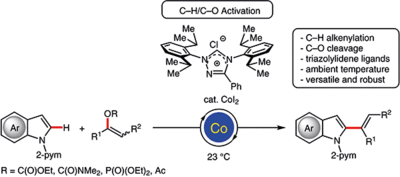
Triazolylidene Ligands Allow Cobalt-Catalyzed C–H/C–O Alkenylations at Ambient Temperature
N. Sauermann, J. Loup, D. Kootz, V. R. Yatham, A. Berkessel, L. Ackermann Synthesis, 2017, 49, 3476–3484. .
DOI: 10.1055/s-0036-1590471
Hydrogen Tunneling Above Room Temperature Evidenced by Infrared Ion Spectroscopy
M. Schäfer, K. Peckelsen, M. Paul, J. Martens, J. Oomens, G. Berden, A. Berkessel, A. Meijer J. Am. Chem. Soc., 2017, 139, 5779–5786.
DOI: 10.1021/jacs.6b10348
Highlighted in J. Am. Chem. Soc. 2017, 139, 6277.
The Catalytic Effect of Fluoroalcohol Mixtures Depends on Domain Formation
O. Holloczki, A. Berkessel, J. Mars, M. Mezger, A. Wiebe, S. R. R. Waldvogel, B. Kirchner ACS Catal., 2017, 7, 1846–1852.
DOI: 10.1021/acscatal.6b03090
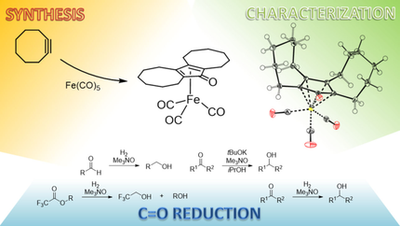
Synthesis of [Bis(hexamethylene)cyclopentadienone]iron Tricarbonyl and its Application to Catalytic Reductions of C=O Bonds
S. Vailati Facchini, J.-M. Neudörfl, L. Pignataro, M. Cettolin, C. Gennari, A. Berkessel, U. Piarulli ChemCatChem, 2017, 8, 1461–1468.
DOI: 10.1002/cctc.201601591
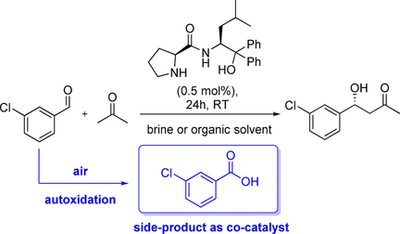
"Side-Product Catalysis": Substrate Autoxidation as an Overlooked Side Reaction Generating a Co-Catalyst for Enhancing Asymmetric Aldol Reactions
M. Heidlindemann, A. Berkessel, H. Gröger ChemCatChem, 2017, 8, 1383–1388.
DOI: 10.1002/cctc.201601530
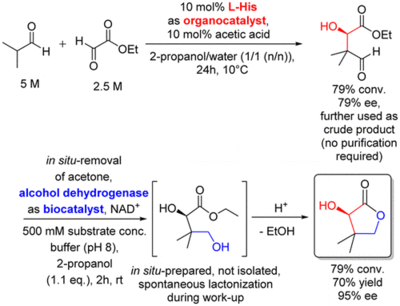
First Tandem-Type One-Pot Process Combining Asymmetric Organo- and Biocatalytic Reactions in Aqueous Media Exemplified for an Enantio- and Diastereoselective Synthesis of 1,3-Diols
G. Rulli, N. Duangdee, W. Hummel, A. Berkessel, H. Gröger Eur. J. Org. Chem., 2017, 4, 812–817.
DOI: 10.1002/ejoc.201600831
Catalytic Prins Reaction Effected by Molecular Iodine in the Presence of Bis(trifluoromethanesulfonyl)imide Salts
W. Harnying, J.-M. Neudörfl, A. Berkessel Synthesis, 2017, 49, 269–274.
DOI: 10.1055/s-0036-1588367
2016
Titanium cis-1,2-Diaminocyclohexane Salalen Catalysts of Outstanding Activity and Enantioselectivity for the Asymmetric Epoxidation of Non-Conjugated Terminal Olefins with H2O2
A. Berkessel, M. Lansing, H. Engler, T. M. Leuther, J. M. Neudörfl ChemCatChem, 2016, 8, 3706–3709.
DOI: 10.1002/cctc.201601154
Screening, Molecular Cloning and Biochemical Characterization of an Alcohol Dehydrogenase from Pichia Pastoris Useful for the Generation of Secondary and Tertiary Alcohols in Enantio- and Diastereomerically Pure Form
D. Bulut, N. Duangdee, H. Gröger, A. Berkessel, W. Hummel ChemBioChem, 2016, 17, 1349–1358.
DOI: 10.1002/cbic.201600101
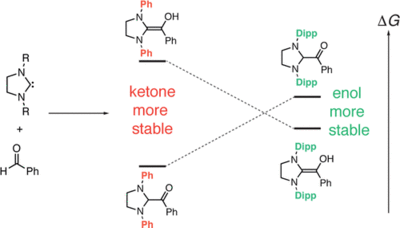
Keto-Enol Thermodynamics of Breslow Intermediates
M. Paul, M. Breugst, J.-M. Neudörfl, R. B. Sunoj, A. Berkessel J. Am. Chem. Soc., 2016, 138, 5044–5051.
DOI: 10.1021/jacs.5b13236
1,4-Bis-Dipp/Mes-1,2,4-Triazolylidenes: Carbene Catalysts that Efficiently Overcome Steric Hindrance in the Redox Esterification of α- and β-Substituted α,β-Enals
V. R. Yatham, W. Harnying, D. Kootz, J.-M. Neudörfl, N. E. Schlörer, A. Berkessel J. Am. Chem. Soc., 2016, 138, 2670–2677.
DOI: 10.1021/jacs.5b11796
Highlighted in Synfacts 2016, 12, 0418.
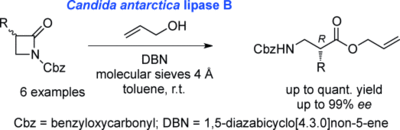
Enantiopure N-Benzyloxycarbonyl-β2-Amino Acid Allyl Esters from Racemic β-Lactams by Enzymatic Dynamic Kinetic Resolution Using Candida antarctica Lipase B
E. Gianolio, R. Mohan, A. Berkessel Adv. Synth. Catal., 2016, 358, 30–33.
DOI: 10.1002/adsc.201500820
2015
N-Heterocyclic Carbene Catalyzed Tail-to-Tail Oligomerization of N,N-Dimethyl-acrylamide (DMAA) and the Search for the Stetter Reaction of DMAA with Benzaldehyde
O.-a. Rajachan, M. Paul, V. R. Yatham, J.-M. Neudörfl, K. Kanokmedhakul, S. Kanokmedhakul, A. Berkessel Tetrahedron Lett., 2015, 56, 6537–6540.
DOI: 10.1016/j.tetlet.2015.09.104
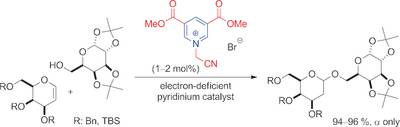
Organocatalytic Glycosylation by Electron Deficient Pyridinium Salts
S. Das, D. Pekel, J.-M. Neudörfl, A. Berkessel Angew. Chem., 2015, 127, 12656–12660; Angew. Chem. Int. Ed., 2015, 54, 12479–12483.
DOI (English Version): 10.1002/anie.201503156
DOI (Deutsche Version): 10.1002/ange.201503156
Highlighted in Synfacts 2015, 11, 1098.
A Theoretical Study of Imine Hydrocyanation by Halogen-Bonding
N. Heinz, M. Dolg, A. Berkessel J. Comp. Chem., 2015, 36, 1812–1817.
DOI: 10.1002/jcc.23999
Carbene Catalyzed Umpolung of α,β-Enals: a Reactivity Study of Diamino Dienols vs. Azolium Enolates, and the Characterization of Advanced Reaction Intermediates
V. R. Yatham, J.-M. Neudörfl, N. E. Schlörer, A. Berkessel Chem. Sci., 2015, 6, 3706–3711.
DOI: 10.1039/C5SC01027F
Chemoenzymatic Synthesis of Vitamin B5-Intermediate (R)‑Pantolactone via Combined Asymmetric Organo- and Biocatalysis
M. Heidlindemann, M. Hammel, U. Scheffler, R. Mahrwald, W. Hummel, A. Berkessel, H. Gröger J. Org. Chem., 2015, 80, 3387–3396.
DOI: 10.1021/jo502667x
Highlighted in ACS Select virtual issue Organocatalysis.
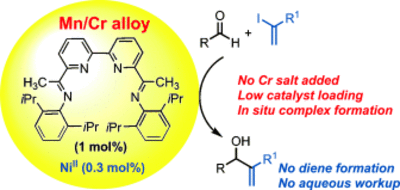
Vinylation of Aldehydes Using Mn/Cr Alloy and a N4-Ligand/Ni(II)-Catalyst
W. Harnying, A. Berkessel Chem. Eur. J., 2015, 21, 6057–6061.
Titanium cis-1,2-Diaminocyclohexane (cis-DACH) Salalen Catalysts for the Asymmetric Epoxidation of Terminal Non-Conjugated Olefins with Hydrogen Peroxide
Q. Wang, J.-M. Neudörfl, A. Berkessel Chem. Eur. J., 2015, 21, 247–254.
Novel Quinone Methide Precursors: Enhanced Sensitivity and Selectivity towards Chronic Lymphocytic Leukemia (CLL) Cells
S. Krallmann, S. Jonas Poll-Wolbeck, H. Flamme, A. Liakos, M. Krüger, A. Berkessel, M. Hallek, K.-A. Kreuzer American Journal of Cancer Research and Clinical Oncology, 2015, 2, 1–25.
2014
Epoxidation of Styrene/Butadiene Star Block Copolymer by Different Methods and Characterization of the Blends with Epoxy Resin
P. Pandit, G. H. Michler, R. Lach, W. Grellmann, J. M. Saiter, A. Berkessel, R. Adhikari Macromol. Symp., 2014, 341, 67–74.
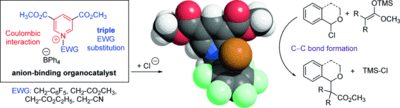
Anion-Binding Catalysis by Electron-Deficient Pyridinium Cations
A. Berkessel, S. Das, D. Pekel, J.-M. Neudörfl Angew. Chem., 2014, 126, 11846–11850; Angew. Chem. Int. Ed., 2014, 53, 11660–11664
DOI (English Version): 10.1002/anie.201403778
DOI (Deutsche Version): 10.1002/ange.201403778
Highlighted in Synfacts 2014, 10, 1330.
Kinetic Resolution of Oxazinones: Rational Exploration of Chemical Space via Design of Experiments
P. Renzi, C. Kronig, A. Carlone, S. Eröksüz, A. Berkessel, M. BellaChem. Eur. J., 2014, 20, 11768–11775.
One-pot Preparation of (S)-N-[(S)-1-Hydroxy-4-methyl-1,1-diphenylpentan-2-yl]pyrrolidine-2-carboxamide from L-Proline
W. Harnying, N. Duangdee, A. Berkessel Org. Synth., 2014, 91, 137–149.
Synthesis of an Oxidation-Stable Analogue of Cyclic Pyranopterin Monosphosphate (cPMP)
S. Hilken, F. Kaletta, A. Heinsch, J.-M. Neudörfl, A. Berkessel Eur. J. Org. Chem., 2014, 11, 2231–2241.
Combination of Asymmetric Organo- and Biocatalytic Reactions in Organic Media Using Immobilized Catalysts in Different Compartments
M. Heidlindemann, G. Rulli, A. Berkessel, W. Hummel, H. Gröger ACS Catal., 2014, 4, 1099–1103
DOI: 10.1021/cs4010387
Highlighted in Synfacts 2014, 10, 428.
On the Involvement of a Spiroepoxide Intermediate in NHC-Catalyzed Benzoin Condensations - an Approach by Oxygenation of Deoxy-Breslow Intermediates
A. Berkessel, S. Elfert Adv. Synth. Catal., 2014, 356, 571–578.
DOI: 10.1002/adsc.201300801
2013
Towards Catalyst Compartimentation in Combined Chemo- and Biocatalytic Processes: Immobilization of Alcohol Dehydrogenases for the Diastereoselective Reduction of a beta-Hydroxy Ketone Obtained from an Organocatalytic Aldol Reaction
G. Rulli, M. Heidlindemann, A. Berkessel, W. Hummel, H. Gröger J. Biotechnol., 2013, 168, 271–276.
DOI: 10.1016/j.jbiotec.2013.08.031
Asymmetric Organocatalytic Aldol Reaction in Water: Mechanistic Insights and Development of a Semi-Continuously Operating Process
G. Rulli, K. A. Fredriksen, N. Duangdee, T. Bonge-Hansen, A. Berkessel, H. Gröger Synthesis, 2013, 48, 2512–2519.
DOI: 10.1055/s-0033-1338509
The Key Intermediates of Carbene-Catalyzed Umpolung Characterized by X-Ray/NMR: Breslow Intermediates, Homoenolates and Azolium Enolates
Charakterisierung der Schlüsselintermediate von carbenkatalysierten Umpolungen durch Kristallstrukturanalyse/NMR-Spektroskopie: Breslow-Intermediate, Homoenolate und Azoliumenolate
A. Berkessel, V. R. Yatham, S. Elfert, J.-M. Neudörfl Angew. Chem. 2013, 125, 11364–11369; Angew. Chem. Int. Ed. 2013, 52, 11158-11162.
DOI (English Version): 10.1002/anie.201303107
DOI (Deutsche Version): 10.1002/ange.201303107
Chiral Ti-Salalen Catalysts Based on cis-1,2-Diaminocyclohexane for the Enantioselective Epoxidation of Terminal, Non-Conjugated Olefins with H2O2 Titan-Salalen-Katalysatoren mit cis-1,2-Diaminocyclohexan-Rückgrat: enantioselektive Epoxidierung terminaler Olefine mit H2O2
A. Berkessel, T. Günther, Q. Wang, J.-M. Neudörfl Angew. Chem. 2013, 125, 8625–8629; Angew. Chem. Int. Ed. 2013, 52, 8467–8471.
DOI (English Verison): 10.1002/anie.201210198
DOI (Deutsche Version): 10.1002/ange.201210198
Severe SMA mice show organ impairment that cannot be rescued by therapy with the HDACi JNJ-26481585
J. Schreml, M. Riessland, M. Paterno, L. Garbes, K. Roßbach, B. Ackermann, J. Krämer, E. Somners, S. H. Parson, R. Heller, A. Berkessel, A. Sterner-Kock, B. Wirth Eur. J. Hum. Genet. 2013, 21, 643–652.
DOI: 10.1038/ejhg.2012.222

Redox Chemistry of Copper Complexes with Various salen Type Ligands
K. Butsch, T. Günther, A. Klein, A. Berkessel, J.-M. Neudörfl Inorg. Chim. Acta 2013, 394, 237–246.
DOI: 10.1016/j.ica.2012.08.016
Dendritic Fluoroalcohols as Catalysts for Alkene Epoxidation with Hydrogen Peroxide (Hot Paper)
Dendritische Fluoralkohole als Katalysatoren für die Epoxidierung von Olefinen mit Wasserstoffperoxid (Hot Paper)
A. Berkessel, J. Krämer, F. Mummy, J.-M. Neudörfl, R. Haag Angew. Chem. 2013, 125, 767–771; Angew. Chem. Int. Ed. 2013, 52, 739-743.
DOI (English Version): 10.1002/anie.201206003
DOI (Deutsche Version): 10.1002/ange.201206003
Highlighted in Synfacts 2013, 9, 451.
2012
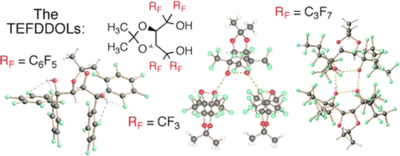
TEFDDOLs (α,α,α',α'-Tetrakis(perfluoroaryl/alkyl)-2,2'-dimethyl-1,3-dioxolane-4,5-dimethanols): Highly Fluorinated Chiral H-Bond Donors and Brønsted Acids with Distinct H-Bonding Patterns and Supramolecular Architectures
A. Berkessel, S. S. Vormittag, N. E. Schlörer, J.-M. Neudörfl J. Org. Chem. 2012, 77, 10145–10157.
DOI: 10.1021/jo301609g
Umpolung by N-Heterocyclic Carbenes: Generation and Reactivity of the Elusive Diaminoenols (Breslow Intermediates)
Umpolung mit N-heterocyclischen Carbenen: Generierung und Reaktivität von Breslow-Intermediaten (2,2-Diaminoenole)
A. Berkessel, S. Elfert, V. R. Yatham, J. Neudörfl, N. Schlörer, J. H. Teles Angew. Chem. 2012, 124, 12537–12541; Angew. Chem. Int. Ed. 2012, 51, 12370–12374.
DOI (English Version): 10.1002/anie.201205878
DOI (Deutsche Version): 10.1002/ange.201205878
Highlighted in Nachr. Chem. 2013, 61, 293.
Highlighted in Synfacts 2013, 5, 105.
Highlighted in Chem. Eng. News 2012, 90, 8.
Highlighted in Nachr. Chem. 2012, 60, 1171.
Highly Enantioselective Organocatalytic Trifluoromethyl Carbinol Synthesis - a Caveat on Reaction Times and Product Isolation
N. Duangdee, W. Harnying, G. Rulli, J.-M. Neudörfl, H. Gröger, A. Berkessel J. Am. Chem. Soc. 2012, 134, 11196–11205.
DOI: 10.1021/ja302511t
Evaluation of the Chiral DIANANE Backbone as Ligand for Organolithium Reagents
J. Praz, L. Guénée, S. Aziz, A. Berkessel, A. Alexakis Adv. Synth. Catal. 2012, 354, 1780–1790.
DOI: 10.1002/adsc.201101016
Enantioselective Self-Sorting on Planar, π-Acidic Surfaces of Chiral Anion-π Transporters
N.-T. Lin, A. V. Jentzsch, L. Guénée, J.-M. Neudörfl, S. Aziz, A. Berkessel, E. Orentas, S. Matile Chem. Sci. 2012, 3, 1121–1127.
DOI: 10.1039/C2SC01013E
Large Scale Synthesis of Singh's Catalyst in a One-Pot Procedure Starting from Proline
A. Berkessel, W. Harnying, N. Duangdee, J.-M. Neudörfl, H. Gröger Org. Proc. Res. Dev. 2012, 16, 123–128.
DOI: 10.1021/op200283q
The L-Leu Hexamer, a Short and Highly Enantioselective Peptide Catalyst for the Juliá-Colonna Epoxidation: Stabilization of a Helical Conformation in DMSO
A. Weyer, D. Diaz, A. Nierth, N. E. Schlörer, A. Berkessel ChemCatChem, 2012, 4, 337–340.
DOI: 10.1002/cctc.201100243
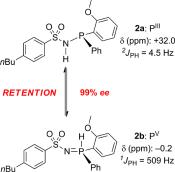
Supramolecular Hydrogen-Bonding Tautomeric Sulfonamido–Phosphinamides: A Perfect P-Chirogenic Memory
F. W. Patureau, M. A. Siegler, A. L. Spek, A. J. Sandee, S. Jugé, S. Aziz, A. Berkessel, J. N. H. Reek Eur. J. Inorg. Chem., 2012, 496–503.
DOI: 10.1002/ejic.201100811
Older Publications
2011
The Antineoplastic Effect of Nitric Oxide-Donating Acetylsalicylic Acid (NO-ASA) in Chronic Lymphocytic Leukemia (CLL) Cells is Highly Dependent on its Positional Isomerism
I. Gehrke, R. Razavi, S. J. Poll-Wolbeck, A. Berkessel, M. Hallek, K.-A. Kreuzer Ther. Adv. Hematol. 2011, 2, 279–289.
DOI: 10.1177/2040620711416272
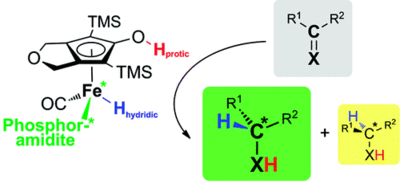
Light-Induced Enantioselective Hydrogenation Using Chiral Derivatives of Casey's Iron-Cyclopentadienone Catalyst
A. Berkessel, S. Reichau, A. von der Höh, N. Leconte, J.-M. Neudörfl Organometallics 2011, 30, 3880–3887.
DOI: 10.1021/om200459s
pKa Values of Chiral Brønsted Acid Catalysts: Phosphoric Acids/Amides, Sulfonyl/Sulfuryl Imides, and Perfluorinated TADDOLs (TEFDDOLs)
P. Christ, A. G. Lindsay, S. S. Vormittag, J.-M. Neudörfl, A. Berkessel, A. C. O'Donoghue Chem. Eur. J. 2011, 17, 8524–8528.
DOI: 10.1002/chem.201101157
Directing Kinetically versus Thermodynamically Controlled Organocatalysis and its Application in Chemoenzymatic Synthesis
Gesteuerte kinetisch oder thermodynamisch kontrollierte Organokatalyse und Anwendung in der chemoenzymatischen Synthese
G. Rulli, N. Duangdee, K. Baer, W. Hummel, A. Berkessel, H. Gröger Angew. Chem. 2011, 123, 8092–8095; Angew. Chem. Int. Ed. 2011, 50, 7944–7949.
DOI (English Version): 10.1002/anie.201008042
DOI (Deutsche Version): 10.1002/ange.201008042
Highlighted in Nat. Chem. 2011, 3, 655.
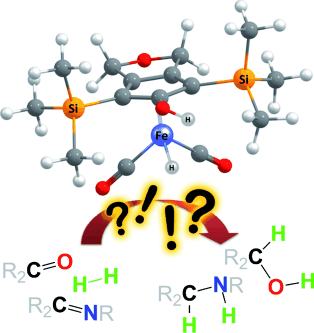
Insight into the Mechanism of Dihydrogen-Heterolysis at Cyclopentadienone Iron Complexes and Subsequent C=X Hydrogenation
A. Berkessel, A. von der Höh ChemCatChem 2011, 3, 861–867.
DOI: 10.1002/cctc.201000428
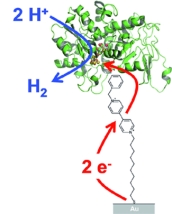
Tailor-Made Modification of a Gold Surface for the Chemical Binding of a High-Activity [FeFe] Hydrogenase
A. Berkessel, H. Krassen, S. T. Stripp, N. Böhm, T. Happe, K. Ataka, J. Heberle Eur. J. Inorg. Chem., 2011, 1138–1146.
DOI: 10.1002/ejic.201001190
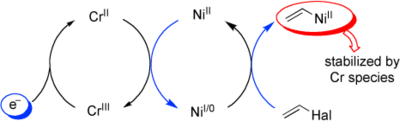
Cr/Ni-Catalyzed Vinylation of Aldehydes: A Mechanistic Study on the Catalytic Roles of Nickel and Chromium
A. Berkessel, W. Harnying, A. Kaiser, A. Klein Chem. Eur. J. 2011, 17, 4765–4773.
DOI: 10.1002/chem.201003366
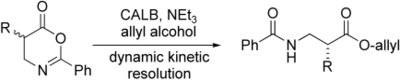
Enzymatic Dynamic Kinetic Resolution of Oxazinones: A New Approach to Enantiopure β2-Amino Acids
A. Berkessel, I. Jurkiewicz, R. Mohan ChemCatChem 2011, 3, 319–330.
DOI: 10.1002/cctc.201000343
2010
Katalytische Wasserstoff-Brücken
A. Berkessel, chemie & more, 2010, 1.10, 12–19.
Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
A. Berkessel in Modern Oxidation Methods, 2nd ed., (Ed.: J. E. Bäckvall), Wiley-VCH, Weinheim, 2010, pp. 117–145.
Chemically Induced Cardiomyogenesis of Mouse Embryonic Stem Cells
A. Berkessel, B. Seelig, S. Schwengberg, J. Hescheler, A. Sachinidis ChemBioChem 2010, 11, 208–217.
DOI: 10.1002/cbic.200900345
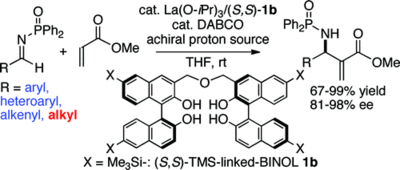
Catalytic Asymmetric Aza-Morita-Baylis-Hillman Reaction of Methyl Acrylate: Role of a Bifunctional La(OiPr)3/Linked-BINOL Complex
T. Yukawa, B. Seelig, Y. Xu, H. Morimoto, S. Matsunaga, A. Berkessel, M. Shibasaki
J. Am. Chem. Soc. 2010, 132, 11988–11992.
DOI: 10.1021/ja103294a
Enantiopure Monoprotected cis-1,2-Diaminocyclohexane: One-Step Preparation and Application in Asymmetric Organocatalysis
A. Berkessel, M.-C. Ong, M. Nachi, J.-M. Neudörfl ChemCatChem 2010, 2, 1215–1218.
DOI: 10.1002/cctc.201000104
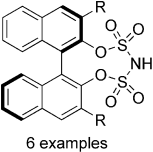
Synthesis and Structural Characterization of a New Class of Strong Chiral Brønsted Acids: 1,1'-Binaphthyl-2,2'-bis(sulfuryl)imides (JINGLEs)
A. Berkessel, P. Christ, N. Leconte, J.-M. Neudörfl, M. Schäfer Eur. J. Org. Chem. 2010, 5165–5170.
DOI: 10.1002/ejoc.201000810

Aldehyde Umpolung by N-Heterocyclic Carbenes: NMR Characterization of the Breslow Intermediate in its Keto Form, and a Spiro-Dioxolane as the Resting State of the Catalytic System
Umpolung von Aldehyden mit N-heterocyclischen Carbenen: NMR-Charakterisierung des Breslow-Intermediats in seiner Ketoform und eines Spirodioxolans als “resting state” des katalytischen Systems
A. Berkessel, S. Elfert, K. Etzenbach-Effers, J. H. Teles Angew. Chem. 2010, 112, 7275–7279; Angew. Chem. Int. Ed. 2010, 49, 7120–7124.
DOI (English Version): 10.1002/anie.200907275
DOI (Deutsche Version): 10.1002/ange.200907275
2009
Noncovalent Organocatalysis Based on Hydrogen Bonding: Elucidation of Reaction Paths by Computational Methods
K. Etzenbach-Effers, A. Berkessel in Asymmetric Organocatalysis (Ed: B. List), Topics Curr. Chem. 2009, 291, pp. 38–69.
Peroxides
A. Berkessel (Volume Editor), Science of Synthesis, Volume 38 , 2009, Thieme, Stuttgart.
Computational Studies of Organocatalytic Processes Based on Hydrogen Bonding
A. Berkessel, K. Etzenbach-Effers in Hydrogen Bonding in Organic Synthesis (Ed.: P. Pihko), Wiley-VCH, Weinheim, 2009, pp. 15–42.
In vivo Role of Cytochrome P450 2E1 and Glutathione-S-Transferase Activity for Acrylamide Toxicokinetics in Humans
O. Doroshyenko, U. Fuhr, D. Kunz, D. Frank, M. Kinzig, A. Jetter, Y. Reith, A. Lazar, D. Taubert, J. Kirchheiner, M. Baum, G. Eisenbrand, F.-I. Berger, D. Bertow, A. Berkessel, F. Sörgel, E. Schömig, D. Tomalik-Scharte Cancer Epidemiol., Biomarkers Prev. 2009, 18, 433–443.
DOI: 10.1158/1055-9965.EPI-08-0832
A Simplified Synthesis of Takemoto's Catalyst
A. Berkessel, B. Seelig Synthesis 2009, 2113–2115.
DOI: 10.1055/s-0029-1216804
Sequential and Modular Synthesis of Chiral 1,3-Diols with Two Stereogenic Centers: Access to All Four Stereoisomers by Combination of Organo- and Biocatalysis
Sequenzielle und modulare Synthese von chiralen 1,3-Diolen mit zwei Stereozentren: Zugang zu allen vier Stereoisomeren durch Kombination von Organo- und Biokatalyse
K. Baer, M. Kraußer, E. Burda, W. Hummel, A. Berkessel, H. Gröger Angew. Chem. 2009, 121, 9519–9522; Angew. Chem. Int. Ed. 2009, 48, 9355–9358.
DOI (English Version): 10.1002/anie.200900582
DOI (Deutsche Version): 10.1002/ange.200900582
Highlighted in Synfacts 2010, 99.
2008
Asymmetric Epoxidation of Olefins with Hydrogen Peroxide - Catalysis by an Aspartate-Containing Tripeptide
Asymmetrische Epoxidierung von Olefinen mit Wasserstoffperoxid – Katalyse durch ein Aspartat enthaltendes Tripeptid
A. Berkessel Angew. Chem. 2008, 120, 3735–3737; Angew. Chem. Int. Ed. 2008, 47, 3677–3679.
DOI (English Version): 10.1002/anie.200705326
DOI (Deutsche Version): 10.1002/ange.200705326
Organocatalysis by Hydrogen Bonding Networks
A. Berkessel in Organocatalysis (Eds: M. T. Reetz, B. List, S. Jaroch, H. Weinmann) Ernst Schering Foundation Symposium Proceedings 2007-2, Springer, Berlin, 2008, 281–297.
DOI: 10.1007/2789_2008_080
Ein Netz aus H-Brücken
A. Berkessel Nachr. Chem. 2008, 56, 126–130.
DOI: 10.1002/nadc.200851968
Mass-Spectrometrical and Kinetic Studies on the Mechanism and Degradation Pathways of Titanium Salalen Catalysts for Asymmetric Epoxidation with Aqueous Hydrogen Peroxide
A. Berkessel, M. Brandenburg, M. Schäfer Adv. Synth. Catal. 2008, 350, 1287–1294.
DOI: 10.1002/adsc.200700601
A Chemical Genetics Approach for Specific Differentiation of Stem Cells to Somatic Cells: A New Promising Therapeutical Approach
A. Berkessel, A. Sachinidis, S. Isaia, B. Seelig, J. Hescheler Comb. Chem. High Throughput Screening 2008, 11, 70–82.
DOI: 10.2174/138620708783398322
2007
Catalytic Asymmetric Epoxidation of Enones and Related Compounds
A. Berkessel in Asymmetric Synthesis - The Essentials (Eds: M. Christmann, S. Bräse) Wiley-VCH, Weinheim, 2007, pp. 185-189.
Stereoselective Michael Addition of Carbonyl Compounds to beta-Nitrostyrene Catalyzed by N-Toluenesulfonyl-L-proline Amide in Ionic Liquids
A. Berkessel, M. Meciarova, K. Hubinska, S. Toma, B. Koch Monatsh. Chem. 2007, 138, 1181–1186.
DOI: 10.1007/s00706-007-0732-0
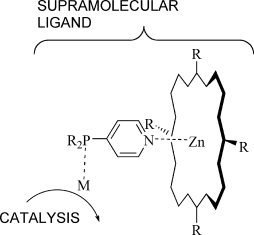
Fine-Tuning Ligands for Catalysis Using Supramolecular Strategies
A. Berkessel, V. F. Slagt, P. Kaiser, M. Kuil, P. W. N. M. van Leeuwen, J. N. H. Reek Eur. J. Inorg. Chem., 2007, 4653–4662.
DOI: 10.1002/ejic.200700550
Practical Two-Step Synthesis of an Enantiopure Aliphatic Terminal (S)-Epoxide Based on Reduction of Haloalkanones with Designer Cells
A. Berkessel, C. Rollmann, F. Chamouleau, S. Labs, O. May, H. Gröger Adv. Synth. Catal. 2007, 349, 2697–2704.
DOI: 10.1002/adsc.200700244
A Practical and Versatile Access to Dihydrosalen (Salalen) Ligands: Highly Enantioselective Titanium In Situ Catalysts for Asymmetric Epoxidation with Aqueous Hydrogen Peroxide
A. Berkessel, M. Brandenburg, E. Leitterstorf, J. Frey, J. Lex, M. Schäfer Adv. Synth. Catal. 2007, 349, 2385–2391.
DOI: 10.1002/adsc.200700221
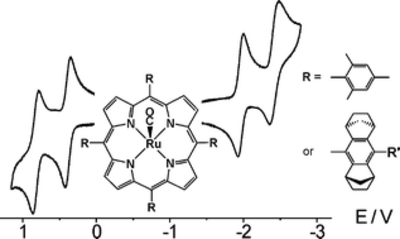
On the Redox States of Ruthenium Porphyrin Oxidation Catalysts
A. Berkessel, E. Ertürk, P. Kaiser, A. Klein, R. M. Kowalczyk, B. Sarkar Dalton Trans., 2007, 3427–3434.
DOI: 10.1039/b705078j
Highly Enantioselective Epoxidation of 2-Methylnaphthoquinone (Vitamin K3) Mediated by New Cinchona Alkaloid Phase-Transfer Catalysts
A. Berkessel, M. Guixa, F. Schmidt, J. M. Neudörfl, J. Lex Chem. Eur. J. 2007, 13, 4483–4498.
DOI: 10.1002/chem.200600993
Toxicokinetics of Acrylamide in Humans after Ingestion of a Defined Dose in a Test Meal to Improve Risk Assessment for Acrylamide Carcinogenicity
U. Fuhr, M. I. Boettcher, M. Kinzig-Schippers, A. Weyer, A. Jetter, A. Lazar, D. Taubert, D. Tomalik-Scharte, P. Pournara, V. Jakob, S. Harlfinger, T. Klaassen, A. Berkessel, J. Angerer, F. Sörgel, E. Schömig Cancer Epidemiol. Biomarkers Prev. 2006, 15, 266–271.
Hydrolytic Kinetic Resolution of Epoxides Catalyzed by Chromium(III)-endo,endo-2,5-diaminonorbornane-salen [Cr(III)-DIANANE-salen] Complexes. Inproved Activity, Low Catalyst Loading
A. Berkessel, E. Ertürk Adv. Synth. Catal. 2006, 348, 2619–2625.
DOI: 10.1002/adsc.200606181
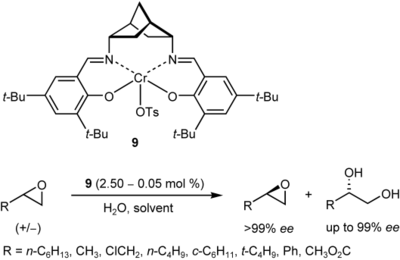
Structural optimization of thiourea-based bifunctional organocatalysts for the highly enantioselective dynamic kinetic resolution of azlactones
A. Berkessel, S. Mukherjee, T. N. Müller, F. Cleemann, K. Roland, M. Brandenburg, J.-M. Neudörfl, J. Lex Org. Biomol. Chem. 2006, 4, 4319–4330.
DOI: 10.1039/b607574f
Enantiomerically Pure Isophorone Diamine [3-(Aminomethyl)-3,5,5-trimethylcyclohexylamine]: A Chiral 1,4-Diamine Building Block Made Available on Large Scale
A. Berkessel, K. Roland, M. Schröder, J. M. Neudörfl, J. Lex J. Org. Chem. 2006, 71, 9312–9318.
DOI: 10.1021/jo0613737
Enantioselective Aldol Reactions Catalysed by N-Toluenesulfonyl-L-proline Amide in Ionic Liquids
M. Meciarová, S. Toma, A. Berkessel, B. Koch Lett. Org. Chem. 2006, 3, 437–441.
DOI: 10.2174/157017806777828402
Asymmetric Morita-Baylis-Hillmann Reaction catalyzed by Isophoronediamine-Derived Bis(thio)urea Organocatalysts
A. Berkessel, K. Roland, J. M. Neudörfl Org. Lett. 2006, 8, 4195–4198.
DOI: 10.1021/ol061298m
Dramatic Acceleration of Olefin Epoxidation in Fluorinated Alcohols: Activation of Hydrogen Peroxide by Multiple H-Bond Networks
A. Berkessel, J. A. Adrio J. Am. Chem. Soc. 2006, 128, 13412–13420.
DOI: 10.1021/ja0620181
Dramatic Enhancement of Enone Epoxidation Rates in Nonionic Microemulsions
T. Wielpütz, T. Sottmann, R. Strey, F. Schmidt, A. Berkessel Chem. Eur. J. 2006, 12, 7565–7575.
DOI: 10.1002/chem.200600550

Catalytic Asymmetric Addition of Carbon Dioxide to Propylene Oxide with Unprecedented Enantioselectivity
A. Berkessel, M. Brandenburg Org. Lett. 2006, 8, 4401–4404.
DOI: 10.1021/ol061501d
Unveiling the "Booster Effect" of Fluorinated Alcohol Solvents: Aggregation-Induced Conformational Changes, and Cooperatively Enhanced H-Bonding
A. Berkessel, J. A. Adrio, D. Hüttenhain, J. M. Neudörfl J. Am. Chem. Soc. 2006, 128, 8421–8426.
DOI: 10.1021/ja0545463

Lipase/Aluminum-Catalyzed Dynamic Kinetic Resolution of Secondary Alcohols
Lipase/Aluminium-katalysierte dynamische kinetische Racematspaltung von sekundären Alkoholen
A. Berkessel, L. Sebastián, T. N. Müller Angew. Chem. 2006, 118, 6717–6720; Angew. Chem. Int. Ed. 2006, 45, 6567–6570.
DOI: 10.1002/anie.200600379
DOI: 10.1002/ange.200600379
Highlighted in Synfacts 2006, 1176.

DIANANE-Cr(III)-salen Complexes as Highly Enantioselective Catalysts for Hetero-Diels-Alder Reactions of Aldehydes with Dienes
A. Berkessel, N. Vogl Eur. J. Org. Chem. 2006, 5029–5035.
DOI: 10.1002/ejoc.200600359
Synthetic Uses of Peroxides
A. Berkessel, N. Vogl in The Chemistry of Peroxides (Ed.: Z. Rappoport), Wiley-VCH, Weinheim, 2006, pp. 307–596.
Asymmetric Enone Epoxidation by Short Solid-Phase Bound Peptides: Further Evidence for Catalyst Helicity and Catalytic Activity of Individual Peptide Strands
A. Berkessel, B. Koch, C. Toniolo, M. Rainaldi, Q. B. Broxterman, B. Kaptein Biopolymers: Pept. Sci. 2006, 84, 90–96.
DOI: 10.1002/bip.20413
Reversal of Enantioselectivity by Catalyst Protonation: Asymmetric Hydrocyanation of Imines with Oxazaborolidines
A. Berkessel, S. Mukherjee, J. Lex Synlett, 2006, 41–44.
DOI: 10.1055/s-2005-922757
Chiral Cr(III) Porphyrins as Highly Enantioselective Catalysts for Hetero-Diels-Alder Reactions Between Aldehydes and Dienes
A. Berkessel, E. Ertürk, C. Laporte Adv. Synth. Catal. 2006, 348, 223–228.
DOI: 10.1002/adsc.200505249
Highlighted in: Synfacts 2006, 358.

Diversity-Based Approaches to Selective Biomimetic Oxidation Catalysis
A. Berkessel Adv. Inorg. Chem. 2006, 58, 1–28.
DOI: 10.1016/S0898-8838(05)58001-2
Kinetic Resolution of Oxazinones: An Organocatalytic Approach to Enantiomerically Pure β-Amino Acids.
Kinetische Racematspaltung von Oxazinonen – ein organokatalytischer Zugang zu enantiomerenreinen β-Aminosäuren
A. Berkessel, F. Cleemann, S. Mukherjee Angew. Chem. 2005, 117, 7632–7635; Angew. Chem. Int. Ed. 2005, 44, 7466–7469.
DOI (English Version): 10.1002/anie.200502003
DOI (Deutsche Version): 10.1002/ange.200502003
Highlighted in: Synfacts 2006, 121.
Combinatorial Approaches to Functional Models for Galactose Oxidase
A. Berkessel, M. Dousset, S. Bulat, K. Glaubitz Biol. Chem. 2005, 386, 1035–1041. DOI: 10.1515/BC.2005.119
Biomimetic and Organocatalytic Approaches to Oxidation Catalysis
A. Berkessel Pure Appl. Chem. 2005, 77, 1277–1284.
DOI: 10.1351/pac200577071277
Organogel Media for On-Bead Screening in Combinatorial Catalysis
K.-J. Johansson, M. R. M. Andreae, A. Berkessel, A. P. Davis Tetrahedron Lett. 2005, 46, 3923–3926.
DOI: 10.1016/j.tetlet.2005.03.125
Second-Generation Organocatalysts for the Highly Enantioselective Dynamic Kinetic Resolution of Azlactones
A. Berkessel, S. Mukherjee, F. Cleemann, T.N. Müller, J.Lex Chem. Commun. 2005, 1898–1900.
DOI: 10.1039/b418666d
Highly Efficient Dynamic Kinetic Resolution of Azlactones by Urea-Based Bifunctional Organocatalysts
Bifunktionale Organokatalysatoren auf Harnstoff-Basis für die effiziente dynamische kinetische Racemattrennung von Azlactonen
A. Berkessel, F. Cleemann, S. Mukherjee, T. N. Müller, J. Lex Angew. Chem. 2005, 117, 817–821; Angew. Chemie Int. Ed. 2005, 44, 807–811.
DOI (English Version): 10.1002/anie.200461442
DOI (Deutsche Version): 10.1002/ange.200461442
Asymmetric Organocatalysis
A. Berkessel, H. Gröger, Wiley-VCH, Weinheim 2005.
rac-Bis[μ-N-salicylidene-N'-(α-phenylsulfanylmethyl-2-oxidobenzyl)propylenediamine-N,N',O,O,O']nickel(II) dichloromethane solvate
A. Berkessel, M. Bolte Acta Cryst. 2004, E60, m387–m389.
DOI: 10.1107/S1600536804004611
Synthesis of Novel 11-Desmethyl Analogues of Laulimalide by Nozaki-Kishi-Coupling
I. Paterson, H. Bergmann, D. Menche, A. Berkessel Org. Lett. 2004, 6, 1293–1295.
DOI: 10.1021/ol049791q
Enantioselective Synthesis of DIANANE, a Novel C2-Symmetric Chiral Diamine for Asymmetric Catalysis
A. Berkessel, M. Schröder, C. A. Sklorz, S. Tabanella, N. Vogl, J. Lex, J. M. Neudörfl J. Org. Chem. 2004, 69, 3050–3056.
DOI: 10.1021/jo035841d
Kinetic Studies of Olefin Epoxidation with Hydrogen Peroxide in 1,1,1,3,3,3-Hexafluoro-2-propanol Reveal a Crucial Catalytic Role for Solvent Clusters
A. Berkessel, J. A. Adrio Adv. Synth. Catal. 2004, 346, 275–280.
DOI: 10.1002/adsc.200303222
Proline-Derived N-Sulfonylcarboxamides: Readily Available, Highly Enantioselective and Versatile Catalysts for Direct Aldol Reactions
A. Berkessel, B. Koch, J. Lex Adv. Synth. Catal. 2004, 346, 1141–1146.
DOI: 10.1002/adsc.200404126
Simple Amino Acids and Short-Chain Peptides as Efficient Metal-Free Catalysts in Asymmetric Synthesis
H. Gröger, J. Wilken, A. Berkessel in Organic Synthesis Highlights V (Eds: H.-G. Schmalz, T. Wirth), Wiley-VCH, Weinheim, 2003, pp. 178–186.
Discovery of Novel Homogeneous Rare Earth Catalysts by IR-Thermography: Epoxide Opening and Baeyer-Villiger Oxidations with Hydrogen Peroxide
A. Berkessel, E. Ashkenazi, M. R. M. Andreae Appl. Catal. A: General, 2003 254, 27–34.
DOI: 10.1016/S0926-860X(03)00260-6
Eletronically Tuned Chiral Ruthenium Porphyrines: Extremely Stable and Selective Catalysts for Asymmetric Epoxidation and Cyclopropanation
A. Berkessel, P. Kaiser, J. Lex Chem. Eur. J. 2003, 9, 4746–4756.
DOI: 10.1002/chem.200305045
Conformational Analysis by HRMAS NMR Spectroscopy of Resin-Bound Homo-Peptides from Cα-Methyl-Leucine
M. Rainaldi, N. Lancelot, K. Elbayed, J. Raya, M. Piotto, J.-P. Briand, B. Kaptein, Q.B. Broxterman, A. Berkessel, F. Formaggio, C. Toniolo, A. Bianco Org. Biomol. Chem. 2003, 1, 1835–1837.
DOI: 10.1039/b303193d
The Discovery of Catalytically Active Peptides through Combinatorial Chemistry
A. Berkessel Curr. Opin. Chem. Biol. 2003, 7, 409–419.
DOI: 10.1016/S1367-5931(03)00065-6
A Highly Enantioselective Catalyst for the Asymmetric Nozaki-Hyama-Kishi Reaction of Allylic and Vinylic Halides
A. Berkessel, D. Menche, C. Sklorz, M. Schröder, I. Paterson Angew. Chem. 2003, 115, 1062–1065; Angew. Chem. Int. Ed. 2003, 42, 1032–1035.
DOI (English Version): 10.1002/anie.200390265
DOI (Deutsche Version): 10.1002/ange.200390240
Baeyer-Villiger-Oxidations with Hydrogen Peroxide in Fluorinated Alcohols: Lactone Formation by a Nonclassical Mechanism
Baeyer-Villiger-Oxidation mit Wasserstoffperoxid in fluorierten Alkoholen: Lactonbildung nach einem nichtklassischen Mechanismus
A. Berkessel, M. R. M. Andreae, H. Schmickler, J. Lex Angew. Chem. 2002, 114, 4661–4664; Angew. Chem. Int. Ed. 2002, 41, 4481–4484.
DOI (English Version): 10.1002/1521-3773(20021202)41:23<4481::AID-ANIE4481
DOI (Deutsche Version): 10.1002/1521-3757(20021202)114:23<4661::AID-ANGE4661
Enantiomerically Pure β-Amino Acids: A Convenient Access to Both Enantiomers of trans-2-Aminocyclohexanecarboxylic Acid
A. Berkessel, K. Glaubitz, J. Lex Eur. J. Org. Chem. 2002, 2948–2952.
DOI: 10.1002/1099-0690(200209)2002:17<2948::AID-EJOC2948
Synthesis and Configurational Assignment of Chiral Salicylic Aldehydes: Novel Building Blocks for Asymmetric Catalysis
A. Berkessel, M. R. Vennemann, J. Lex Eur. J. Org. Chem., 2002, 2800–2807.
DOI: 10.1002/1099-0690(200208)2002:16<2800::AID-EJOC280
Nonionic Microemulsions with Chlorinated Hydrocarbons for Catalysis
H. Egger, T. Sottmann, R. Strey, C. Valero, A. Berkessel Tenside, Surfactants, Detergents 2002, 39, 17–22.
Hydrogenation without a Transition-Metal Catalyst: On the Mechanism of the Base-Catalyzed Hydrogenation of Ketones
A. Berkessel, T. J. S. Schubert, T. N. Müller J. Am. Chem. Soc. 2002, 124, 8693–8698.
DOI: 10.1021/ja016152r
Highly Enantioselective Enone Epoxidation Catalyzed by Short Solid Phase-Bound Peptides: Dominant Role of Peptide Helicity
A. Berkessel, N. Gasch, K. Glaubitz, C. Koch Org. Lett. 2001, 3, 3839–3842.
DOI: 10.1021/ol0166451
Activation of dihydrogen without transition metals
A. Berkessel Curr. Opinion Chem. Biol. 2001, 5, 486–490.
DOI: 10.1016/S1367-5931(00)00245-3
Biomimetic Oxidation of Organic Substrates with Hydrogen Peroxide
A. Berkessel TCI Mail 2001, 109, 3–13.
Efficient catalytic methods for the Baeyer-Villiger oxidation and epoxidation with hydrogen peroxide
A. Berkessel, M. R. M. Andreae Tetrahedron Lett. 2001, 42, 2293–2295.
DOI: 10.1016/S0040-4039(01)00141-1
Chiral pentaccordinated Manganese Complexes as Biomimetic Catalysts for Asymmetric Epoxidations with Hydrogen Peroxide
A. Berkessel, T. Schwenkreis, M. Frauenkron, A. Steinmetz, N. Schätz, J. Prox in Peroxide Chemistry (Ed: W. Adam) Wiley-VCH, Weinheim, 2000, pp. 511–525.
Photochemistry of 4'-Benzophenone-Substituted Nucleoside Derivatives as Models for Ribonucleotide Reductases: Competing Generation of 3'-Radicals and Photoenols
T. E. Lehmann, G. Müller, A. Berkessel J. Org. Chem. 2000, 65, 2508–2516.
DOI: 10.1021/jo991811s
Combinatorial de novo Synthesis of Catalysts - How Much of Hit-Structure is Needed for Activity?
A. Berkessel, R. Riedl J. Comb. Chem. 2000, 2, 215–219.
DOI: 10.1021/cc990073i
Mn-Trimethyltriazacyclononane/Ascorbic Acid: A Remarakably Efficient Catalyst for the Epoxidation of Olefins and the Oxidation of Alcohols with Hydrogen Peroxide
A. Berkessel, C. A. Sklorz Tetrahedron Lett. 1999, 40, 7965–7968.
DOI: 10.1016/S0040-4039(99)01595-6
Discovery of Peptide-Zirconium Complexes that Mediate Phosphate Hydrolysis by Batch Screening of a Combinatorial Undecapeptide Library
Identifizierung von Peptid-Zirconium-Komplexen, die die Phosphathydrolyse beschleunigen, durch „On-bead-screening” einer kombinatorischen Undecapeptid-Bibliothek
A. Berkessel, D. A. Hérault Angew. Chem. 1999, 111, 99–102; Angew. Chem. Int. Ed. 1999, 38, 102–105.
DOI (english Version): 10.1002/(SICI)1521-3773(19990115)38:1/2<102::AID-ANIE102>3.0.CO;2-H
DOI (Deutsche Version): 10.1002/(SICI)1521-3757(19990115)111:1/2<99::AID-ANGE99>3.0.CO;2-Q
Transition Metal Complexes as Models for Metallo Enzymes: Mechanistic Studies and Preparative Application
A. Berkessel in Selective Reactions of Metal-Activated Molecules (Eds: H. Werner, P. Schreier) Vieweg, Wiesbaden, 1998, pp. 25–33.
Activation and Thermostabilization Effects of Cyclic 2,3-Diphosphoglycerate on Enzymes from the Hyperthermophilic Methanopyrus kandleri
S. Shima, D. A. Hérault, A. Berkessel, R. K. Thauer Arch. Microbiol. 1998, 170, 469–472.
DOI: 10.1007/s002030050669
Structure Elucidation and Chemical Synthesis of Stigmolone, a Novel Type of Procaryotic Pheromone
W. E. Hull, A. Berkessel, W. Plaga Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 11268–11275.
Probing the Scope of the Asymmetric Dihydroxylation of Polymer-Bound Olefins. Monitoring by HRMAS NMR Allows for Reaction Control and On-Bead Measurement of Enantiomeric Excess
R. Riedl, R. Tappe, A. Berkessel J. Am. Chem. Soc. 1998, 120, 8994–9000.
DOI: 10.1021/ja980183d
Schiff-Base Ligands Carrying Two Elements of Chirality: Matched-Mismatched Effects in the Vanadium Catalyzed Sulfoxidation of Thioethers with Hydrogen Peroxide
A. H. Vetter, A. Berkessel Tetrahedron Lett. 1998, 39, 1741–1744.
DOI: 10.1016/S0040-4039(98)00084-7
Hydrogenation without a Metal Catalyst: An ab initio-Study on the Mechanism of the Metal-Free Hydrogenase from Methanobacterium thermoautotrophicum
J. H. Teles, S. Brode, A. Berkessel J. Am. Chem. Soc. 1998, 120, 1345–1346.
DOI: 10.1021/ja972320x
Model Studies on Methyl Coenzyme M Reductase from Methanogenic Bacteria, Mechanistic Investigations, and Preparative Applications
A. Berkessel Bioinorg. Chem. 1997, 431–445.
Catalytic asymmetric epoxidation with a chiral ruthenium porphyrin and N-oxides
A. Berkessel, M. Frauenkron Perkin Trans. 1, 1997, 2265–2266.
DOI: 10.1039/A704275B
Analysis of Ruthenium-Carbonyl-Porphyrin-Complexes: A Comparison of MALDI-TOF, FAB and FD Mass Spectrometry
M. Frauenkron, A. Berkessel, J. H. Gross Eur. Mass Spectrom. 1997, 3, 427–438.
DOI: 10.1255/ejms.177
A Novel Chiral Ruthenium Porphyrin as Highly Efficient and Selective Catalyst for Asymmetric Cyclopropanations
A. Berkessel, M. Frauenkron Tetrahedron Lett. 1997, 38, 7175–7176.
DOI: 10.1016/S0040-4039(97)01763-2
Fluorescence Reporters for Phosphodiesterase Activity
Fluoreszenz-Reporter für Phosphodiesterase-Aktivität
A. Berkessel, R. Riedl Angew. Chem. 1997, 109, 1518–1520; Angew. Chem. Int. Ed. Engl. 1997, 36, 1481–1483.
DOI (English Version): 10.1002/anie.199714811
DOI (Deutsche Version): 10.1002/ange.19971091313
Nickel(II) Complexes of Chiral Tripodal N,O,S,-Ligands: Square-Planar vs. pseudo-Octahedral Coordination in the Solid State and in Solution, Metal-Induced Racemization of the Ligand
A. Berkessel, J. W. Bats, M. Bolte, T. Neumann, L. Seidel Chem. Ber. 1997, 130, 891–897.
DOI: 10.1002/cber.19971300713
Metal-Free Bacterial Haloperoxidases as Unusual Hydrolases: Activation of H2O2 by the Formation of Peracetic Acid
Metallfreie bakterielle Haloperoxidasen als ungewöhnliche Hydrolasen: Aktivierung von H2O2 durch Bildung von Peressigsäure
M. Picard, J. Gross, E. Lübbert, S. Tölzer, S. Krauss, K.-H. van Pée, A. Berkessel Angew. Chem. 1997, 109, 1245–1248; Angew. Chem. Int. Ed. Engl. 1997, 36, 1196–1199.
DOI (English Version): 10.1002/anie.199711961
DOI (Deutsche Version): 10.1002/ange.19971091118
Stereoselective Synthesis of 4'-Benzophenone-Substituted Nucleoside Analogs: Photoactive Models for Ribonucleotide Reductases
T. E. Lehmann, A. Berkessel J. Org. Chem. 1997, 62, 302–309.
DOI: 10.1021/jo961372m
Pentacoordinated Manganese Complexes as Biomimetic Catalysts for Asymmetric Epoxidations with Hydrogen Peroxide
A. Berkessel, M. Frauenkron, T. Schwenkreis, A. Steinmetz J. Mol. Catal. A 1997, 117, 339–346.
DOI: 10.1016/S1381-1169(96)00361-5
Pentacoordinated Manganese(III) Dihydrosalen Complexes as Biomimetic Oxidation Catalysts
A. Berkessel, M. Frauenkron, T. Schwenkreis, A. Steinmetz, G. Baum, D. Fenske J. Mol. Catal. A 1996, 113, 321–342.
DOI: 10.1016/S1381-1169(96)00116-1
Preparation and X-ray Crystal Structure of the First Trimeric Nickel Thiosemicarbazone Complex: The First Example of Oligomerization by Both Ni-O-Ni and Ni-S-Ni Bridging
A. Berkessel, G. Hermann, O.-T. Rauch, M. Büchner, A. Jacobi, G. Huttner Chem. Ber. 1996, 129, 1421–1423.
DOI: 10.1002/cber.19961291203
Synthesis and X-ray Crystal Structure of the First Mononuclear Nickel(II) Alkane Thiolate Complex with a Mixed (S,N,N,O) Ligand Field
A. Berkessel, M. Bolte, T. Neumann, L. Seidel Chem. Ber. 1996, 129, 1183–1189.
DOI: 10.1002/cber.19961291007
A Synthesis of cyclo-2,3-Diphospho-D-glycerate from D-Mannitol
A. Berkessel, U. Geisel, D. A. Hérault Tetrahedron Lett. 1996, 37, 355–356.
DOI: 10.1016/0040-4039(95)02154-X
Stoichiometric Asymmetric Oxidation with Hydrogen Peroxide Activated by a Chiral Phosphoryl Chloride
A. Berkessel, M. Frauenkron Tetrahedron: Asymmetry 1996, 7, 671–672.
DOI: 10.1016/0957-4166(96)00061-4
Synthesis of Novel Tridentate N,O,S and N,N,O Ligands of the Tripod Type in Racemic and Enantiomerically Pure Form
A. Berkessel, M. Bolte, M. Frauenkron, T. Nowak, T. Schwenkreis, L. Seidel, A. Steinmetz Chem. Ber. 1996, 129, 59–68.
DOI: 10.1002/cber.19961290113
On the Mechanism of Catalysis by a Metal-Free Hydrogenase from Methanogenic Archaea: Enzymatic Transformation of H2 without a Metal and Its Analogy to the Chemistry of Alkanes in Superacidic Solution
Zum Katalysemechanismus einer metallfreien Hydrogenase aus methanogenen Archaea: Enzymatische Umsetzung von H2 ohne Metall und ihre Analogie zur Chemie der Alkane in supersaurer Lösung
A. Berkessel, R. K. Thauer Angew. Chem. 1995, 107, 2418–2421; Angew. Chem. Int. Ed. Engl. 1995, 34, 2247–2250.
DOI (English Version): 10.1002/anie.199522471
DOI (Deutsche Version): 10.1002/ange.19951072011
Nickel Complex Catalyzed Reduction of Imines
A. H. Vetter, A. Berkessel Synthesis, 1995, 419–422.
DOI: 10.1055/s-1995-3924
Air Oxidation of a Manganese(III) Thioether Chelate Affords the First Manganese(III) Dihydrosalen Complex with a Pendant Sulfoxide Ligand
A. Berkessel, M. Bolte, T. Schwenkreis J. Chem. Soc., Chem. Commun., 1995, 535-536.
DOI: 10.1039/C39950000535
Synthesis, X-Ray Crystal Structure and Magnetic Characterization of the First "Ni3S4N3" Nickel Thiolate Cluster
A. Berkessel, J. W. Bats, M. Hüber, W. Haase, T. Neumann, L. Seidel Chem. Ber. 1995, 128, 125–129.
DOI: 10.1002/cber.19951280207